
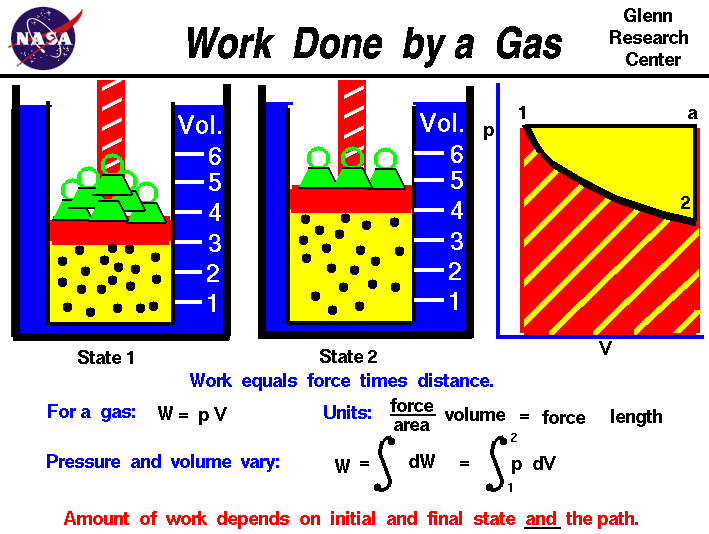
Species not participating in the electrochemical charge transfer reaction only indirectly alter the voltage through the species mole fractions of the participating species.įor the H 2/O 2 fuel cell potential, the open-circuit voltage is the maximum operating voltage (when no current is flowing) and is determined by the chemical thermodynamics of the overall cell reaction. (8) are represented directly in the activity terms of Eq. Only species directly involved in the electrochemical reaction of Eq. A particular gradient can exist between the concentration of a species in the channel of a fuel cell and the electrode, especially under high-current-density conditions, which cannot be considered a true thermodynamic equilibrium situation anyway. The Nernst equation is a result of the equilibrium established at the electrode surfaces. Using this expression, we can solve for the expected maximum (Nernst) voltage for a given fuel cell reaction. Where the partial pressures are evaluated at the particular electrode where the reaction involving the species occurs.
The efficiency of a fuel cell is the useful energy output which is the electrical energy produced, and the energy input is the enthalpy of hydrogen.Į T P = E o − RT nF ln P C P o c P D P o d P A P o a P B P o b E11 The fuel cell performance is examined through the reversible voltage, and the actual output voltage is after overpotential. Specific heat is a measure of the amount of heat energy required to increase the temperature of a substance by 1☌. Change in enthalpy and change in entropy are significant in particular to fuel cells they indicate spontaneity of the adsorption process and increased randomness of adsorbate molecules on the solid surface, respectively.
FIRST LAW HEAT AND WORK IN THERMODYNAMICS CALCULATOR FREE
Gibbs free energy is the thermodynamic potential that measures the reversible work by a thermodynamic system at constant pressure and temperature. The concepts enthalpy, specific heat, entropy and Gibbs free energy are related to the reacting systems in fuel cells. A general thermodynamic analysis of hydrogen fuel cells of the reversible work for the reversible reaction is performed. Hydrogen and oxygen are used to illustrate the simplest case. The amount of work/heat produced depends on thermodynamic values for reversible reactions, while for irreversible reactions overpotential is required to complete the work.
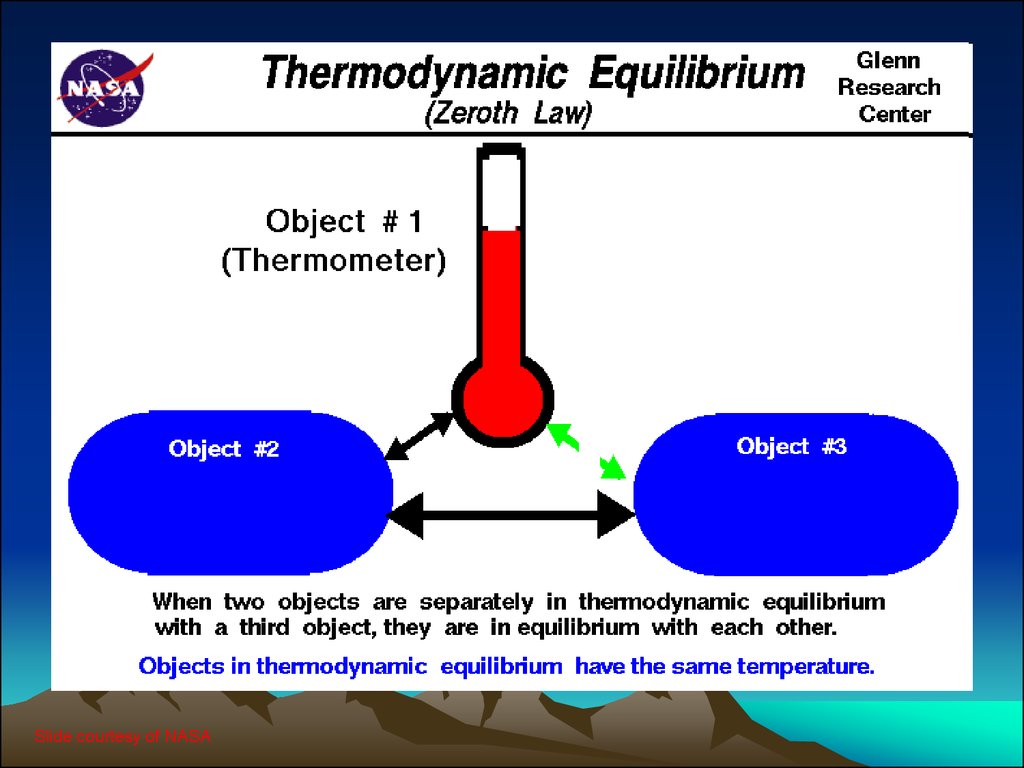
Any system producing energy obeys the laws of thermodynamics. The specific topics explored in this chapter include enthalpy, entropy, specific heat, Gibbs free energy, net output voltage irreversible losses in fuel cells and fuel cell efficiency.įuel cells are electrochemical devices that convert chemical energy into work in the form of electric energy and heat. Basic thermodynamic concepts allow one to predict states of the fuel cell system, including the potential, temperature, pressure, volume and moles of a fuel cell. It also defines how reversible fuel cell voltages, which are the maximum fuel cell performances, are affected by departures from the standard state. This chapter examines how electrical energy and thermal energy are transferred in the hydrogen fuel cell system. Based on the first and second laws of thermodynamics, one can write down thermodynamic potentials to specify how energy can be transferred from one form to another. When a fuel cell is operating, some of the input is used to create electrical energy, but another portion is converted into thermal energy, depending on the type of fuel cell. The predictions that can be made using thermodynamic equations are essential for understanding fuel cell performance, as a fuel cell is an electrochemical device that converts the chemical energy of a fuel and an oxidant gas into electrical energy. Thermodynamics is the study of energy change from one state to another.


 0 kommentar(er)
0 kommentar(er)
